Results Set the Stage for an Upcoming US Trial
January 6, 2021 – Morgan Hill, CA – Mitre Medical Corp., (“Mitre” or “company”) an early-stage medical device company developing the Mitral Touch®, a less invasive and safer approach to treat functional/secondary mitral valve regurgitation (FMR) and remodel the left ventricle (LV), announced the publication of the one-year results of the Mitral Touch in European patients. The article entitled “First in Human Experience with an Epicardial Beating Heart Device for Secondary Mitral Regurgitation” by Thourani et al. was published online December 14, 2020, in the Journal of Thoracic and Cardiovascular Surgery website.
The excerpt below from the discussion section of this peer-reviewed article speaks to the validation of the Mitral Touch in providing cardiac surgeons a beating heart option for these undertreated patients and the unprecedented reduction of these patients’ enlarged ventricles (LVESV reduction). Reducing the left ventricle interrupts the deadly heart failure cycle.
“Epicardial annuloplasty [by the Mitral Touch] provides the opportunity for beating heart evaluation under TEE [ultrasound imaging] guidance of the annuloplasty effect without an atriotomy. Avoiding the atriotomy and extended bypass times may reduce recovery time, atrial arrhythmias, and embolization compared to standard mitral repair. This first-in-man study supports the conclusion that the device improves MR and promotes LV remodeling in patients with secondary MR.
The LV support provided by the epicardial annuloplasty device may explain the improvement in LVESV remodeling (-31%) compared with the remodeling improvement in CTSN Trials (-11.1% and -16.8). at 1-year.”
The structural heart market sees a very rapid uptake for new lower-risk devices that focus on specific patient populations. The Mitral Touch follows this strategy.
As lead author, Dr. Vinod H. Thourani, Marcus Chief of Cardiovascular Surgery for Piedmont Healthcare and the Marcus Heart Valve Center, noted, “We, as cardiac surgeons, are hungry for devices that are less invasive and improve outcomes. These results demonstrate that without opening the heart, we can provide significant MR and left ventricular reduction. The bypass patients with secondary MR represent one of the largest unmet needs in our operating rooms. Presently, these patients go undertreated due to the invasive nature and limited benefit of standard annuloplasty. Confirming the Mitral Touch results in a larger patient trial would be a win for patients and a win for surgeons.”
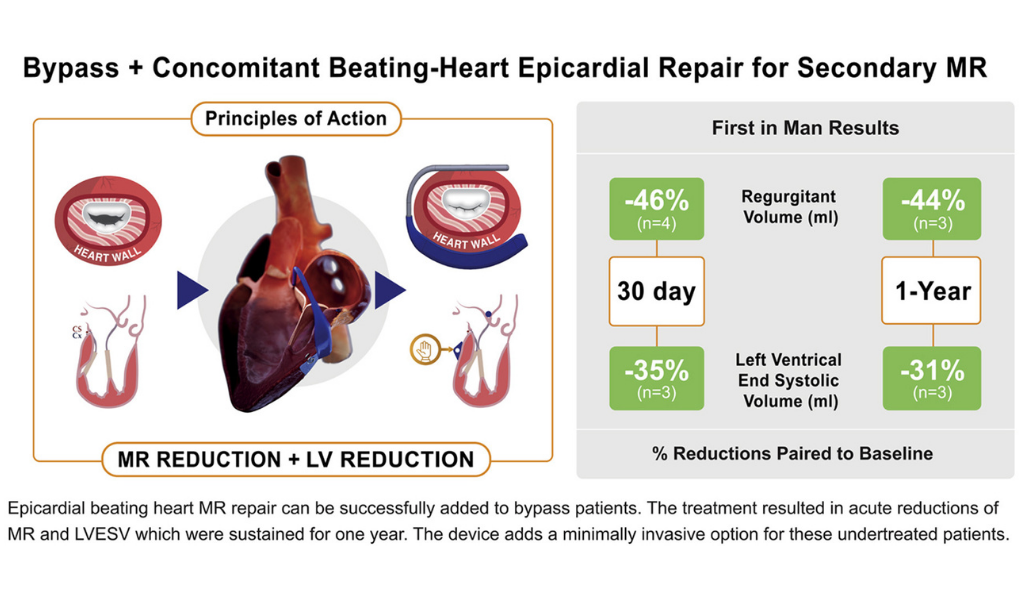
Image description: Epicardial beating heart MR repair can be successfully added to bypass patients. The treatment resulted in acute reductions of MR and LVESV, which were substituted for one year. The device adds a minimally invasive option for these undertreated patients.
Image Source: Thourani, V. (2020). First in Human Experience with an Epicardial Beating Heart Device For Secondary Mitral Regurgitation [image] Journal of Thoracic and Cardiovascular Surgery.
https://marlin-prod.literatumonline.com/cms/attachment/6f39f4fc-501e-479d-8a94-0cd0dee8ba3c/fx1.jpg
“We are pleased that the results were recognized and accepted by the Journal of Thoracic and Cardiovascular Surgery as a valuable contribution to research in the field. The successful treatment of this first set of patients confirms that the Mitral Touch is a non-invasive solution to FMR and remodeling the LV and sets the stage for a larger study in the US,” stated John MacMahon, Chief Executive Officer of Mitre Medical.
About Mitre Medical Corp.
Mitre Medical Corp is an early-stage medical device company developing the Mitral Touch®, a less invasive and safer approach to treat functional mitral valve regurgitation (FMR) and remodel the left ventricle (LV) in patients with moderate to severe MR. Mitre Medical has the potential to be the new standard of care for FMR. For more information, please visit www.mitremedical.com.
Forward-Looking Statements
This press release includes forward-looking statements including, but not limited to, statements related to the development of our technology, our operations, and business strategy, our expected financial results, and corporate updates. The forward-looking statements in this press release are based on management’s current expectations and are subject to substantial risks, uncertainty, and changes in circumstances. Actual results may differ materially from those expressed by these expectations due to risks and uncertainties. Forward-looking statements speak only as of the date of this press release. We undertake no obligation to review or update any forward-looking statement except as may be required by applicable law.
Mitre Medical Corp.:
John MacMahon, Chief Executive Officer
18655 Madrone Parkway
Morgan Hill, CA 95037
Tel: +1 603.264.7751
Email: jmacmahon@mitremedical.com
Investor Contact:
Jennifer K. Zimmons, Ph.D.
Investor Relations
Zimmons International Communications
Tel: +1 917.214.3514
Email: jzimmons@zimmonsic.com
Source: Mitre Medical Corp.

